Beta Lactam Antibiotics
Discovery
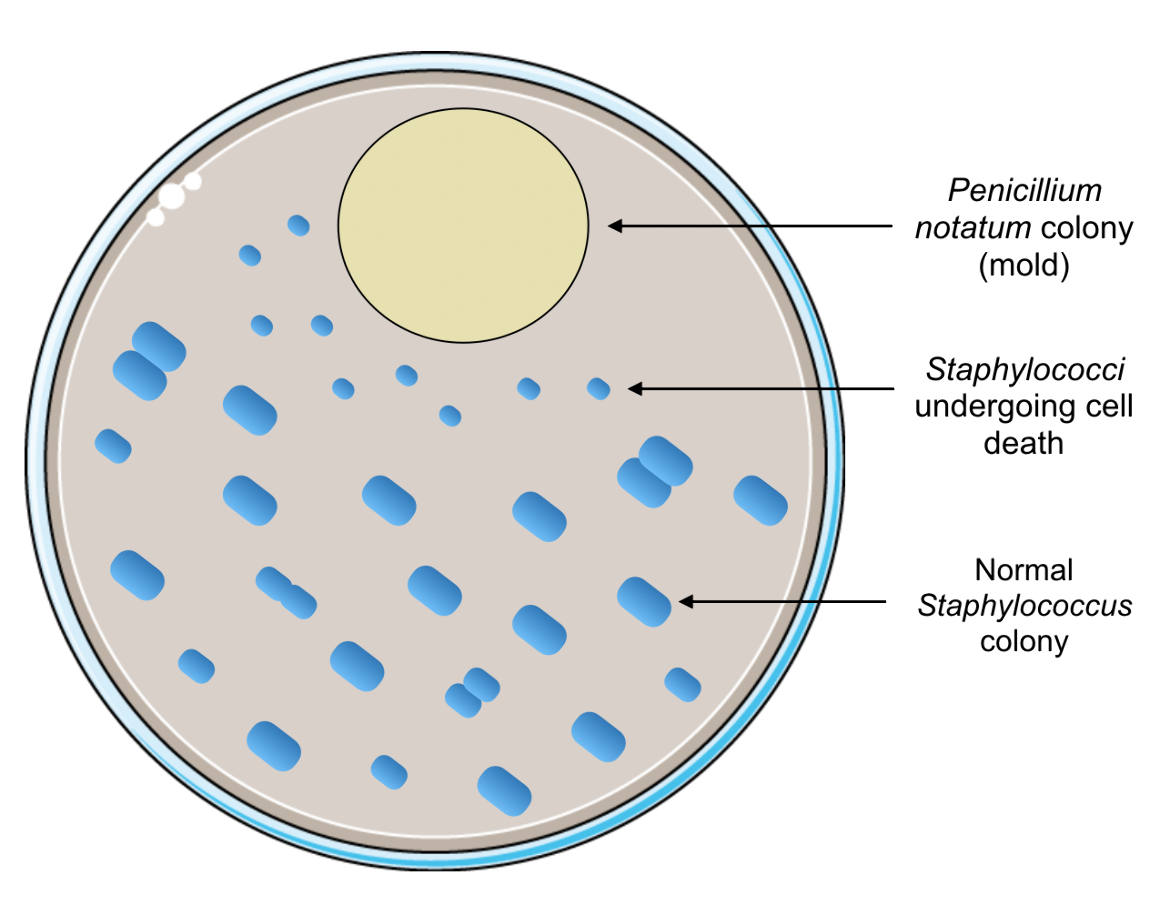
The discovery of one of the first antibiotics, which would become such a huge turning point in the course of medicine, was surprisingly an accident. The story started in London in September of 1928, when Alexander Fleming, a Scottish bacteriologist, was experimenting with the bacteria Staphylococcus aureus. Before he left for vacation, he grew Staphylococcus colonies on culture plates and left them on his laboratory bench. When he returned, he found several of those plates contaminated with mold and initially discarded those plates (Kong et al., 2010). However, the next day he recovered some of those plates to show his experiment to a colleague. Upon close inspection of one of the plates, he saw that the contaminant was preventing the normal growth of Staphylococcus colonies in its proximity. Specifically, these bacterial colonies appeared to be dying (Figure 1), which led Fleming to realize that the mold had antibacterial properties (Kong et al., 2010). Originally, Fleming referred to the mold as Penicillium rubrum, from which he coined the term penicillin. However, it was later identified that the mold was a fungal colony of Penicillium notatum (Kong et al., 2010).
|
| Figure 1. A sample illustration of what Alexander Fleming observed on his contaminated culture plate. Portions of the figure were adapted from Servier Medical Art. |
Although Fleming had uncovered the therapeutic potential of penicillin in 1928, it was not used clinically to treat bacterial infections until the 1940s. This was because Fleming was unable to purify enough penicillin for clinical experiments (Kong et al., 2010). The work of Florey and Chain, as well as other colleagues, allowed the antibiotic to be mass-produced and the antibacterial properties of penicillin to be studied clinically (Kong et al., 2010). The use of penicillin dropped the death rate due to bacterial pneumonia from 18% in World War I to less than 1% during World War II (PBS, 2013). The first American civilian patient, Anne Miller, was successfully treated with penicillin in March of 1942. She had developed blood poisoning (Streptococcal septicemia), due to an infection caused by miscarriage. The discovery of penicillin is said to have revolutionized medicine because its clinical use allowed infections that were previously severe and often even fatal to now be easily treated.
A patch of mold on a culture plate paved the way for an entire class of antibiotics to be discovered or synthesized in the coming years, all of which would become known as beta-lactams or β-Lactams.
Overview of Chemistry
Antibiotics are drugs that treat bacterial infections either by inhibiting bacterial growth and replication or by killing the invading pathogens. Specifically, the class of β-lactams consists of antibiotics that inhibit the bacterial enzymes that catalyze the final step involved in synthesizing peptidoglycan, the major component of the bacterial cell wall. This final step consists of cross-linking peptidoglycan strands to create a mesh-like structure, which provides the cell wall with its structure and rigidity. The bacterial enzymes responsible for this final step were later named penicillin-binding proteins (PBPs) since penicillin and other β-lactam antibiotics are able to bind to them. Disrupting peptidoglycan production compromises the integrity of the cell wall; therefore, when β-lactams inhibit PBPs, the cell wall is severely weakened and can no longer protect the bacterial cell from osmotic damage. As water from the environment continues to move into the cell, increasing osmotic pressure will cause the cell to burst, or lyse. β-lactams are referred to as bactericidal agents because they induce bacterial cell lysis and death. Bacteriostatic agents, on the other hand, prevent bacterial growth and reproduction and may not necessarily kill bacteria.
Click here to learn more about peptidoglycan or cell wall biosynthesis or the Penicillin Binding Protein (or PBP) enzymes.
Where does the Name Beta-Lactam Come From?
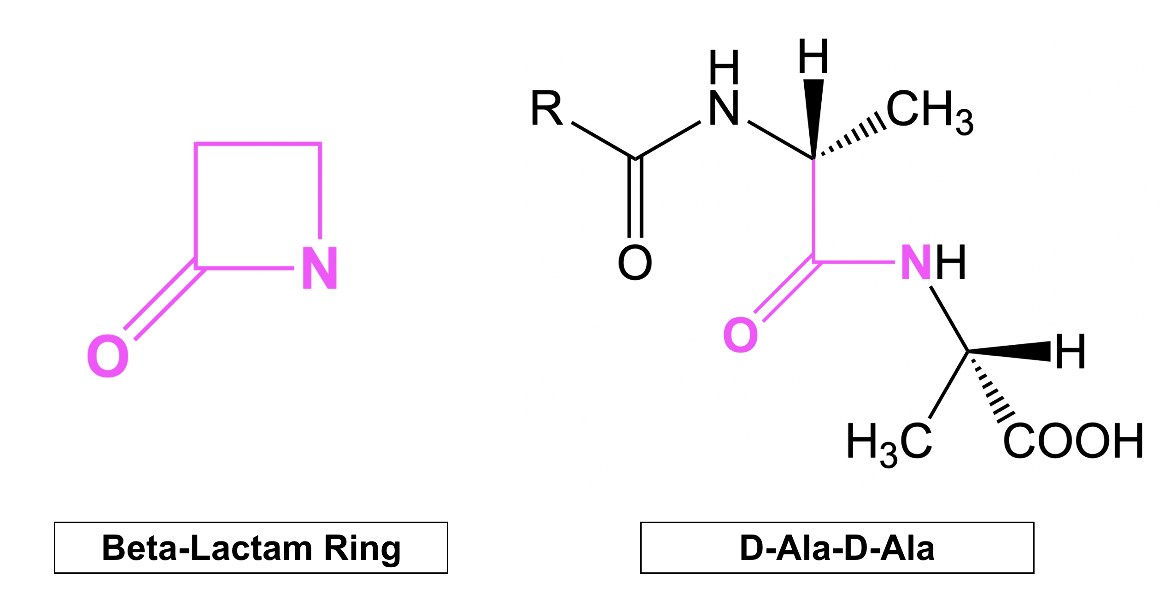
All beta-lactam (henceforth written as β-Lactam) antibiotics feature a highly strained and reactive four-membered ring, known as the β-lactam ring (shown on the left in Figure 1). This core chemical structure is crucial to antibiotic action because of its phenomenal structural similarity to the natural substrate of PBPs. To be more specific, the β-lactam ring system mimics the backbone of the terminal D-alanyl-D-alanine (D-Ala-D-Ala) peptide (Figure 1) of the peptidoglycan strand. Because this peptide sequence serves as the substrate for PBP enzymes during the cross-linking reaction, β-lactam antibiotics can act as substrate analogs and occupy the active site. This keeps the natural substrate from binding and prevents cross-linking and formation of the cell wall peptidoglycan.
Types of β-Lactams
In general, β-lactam antibiotics are divided into the following four subgroups based on structural and clinical differences:
1. Penicillins
2. Cephems
3. Monobactams
4. Carbapenems
Structurally, all four classes of β-lactam antibiotics contain the β-lactam ring; however, they all differ based on the ring structures and substituents that surround that characteristic β-lactam ring. Some may have additional five- or six-membered rings attached, while other β-lactam antibiotics are monocyclic, containing only the β-lactam ring. Since there are a variety of bacterial infections that can be caused by countless types of bacteria, different types of β-lactams need to be synthesized to target unique infections. Clinically, the four subgroups may have different antimicrobial properties, including varying levels of activity (broad or narrow) and the different types of bacteria (either gram positive, gram negative, or both) that are affected. These structural and clinical differences are described herein.
Penicillins
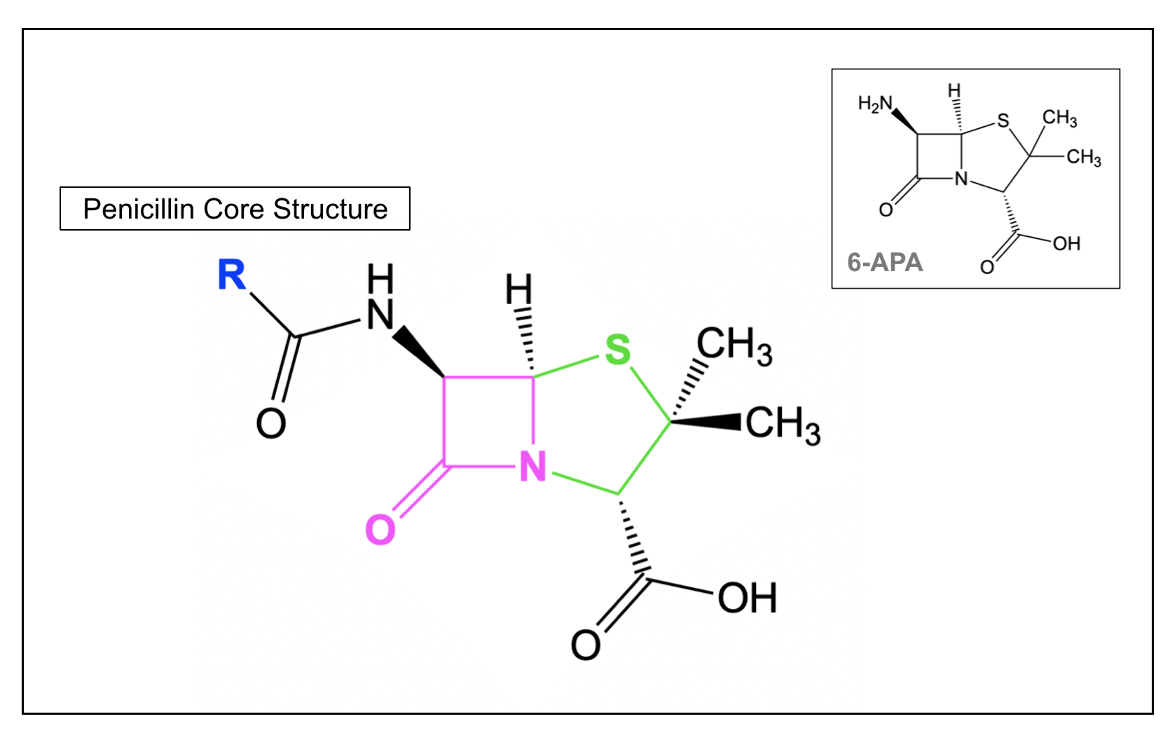
The core structure of penicillins (Figure 3) consists of a β-lactam ring that is attached to a saturated, five-membered ring and a varying side chain (R). All penicillins have a common 6-aminopenicillanic acid (6-APA) nucleus (shown in the inset in Figure 3), which is critical to antibacterial activity (Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e). When the five-membered ring includes a sulfur atom in it (i.e., is a thiazolidine), the β-lactam is classified as a Penicillin. β-lactams, where the sulfur atom is replaced by a carbon are called a carbapenam, while replacing the sulfur with an oxygen forms an oxapenam or clavam.
Note that penicillin can be obtained in large quantities naturally, from Penicillium chrysogenum. With the help of an additional enzyme from P. chrysogenum, the side chain of penicillin can be cleaved to yield 6-aminopenicillanic acid (6-APA), which is shown in the inset on the right in Figure 3. Different side chains can then be added to 6-APA to produce various semisynthetic penicillins (Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e). These side chains, which differ among the various types of penicillin antibiotics, determine some of the unique pharmacological and antibacterial properties of each drug. While Penicillin G and Penicillin V are the only naturally obtained penicillins, used clinically, various semisynthetic penicillin antibiotics, such as methicillin and ampicillin, are also prescribed. Natural penicillins are generally narrow-spectrum and target gram-positive bacteria, with limited activity against gram-negative microbes. Semisynthetic penicillins tend to have more broad-spectrum activity.
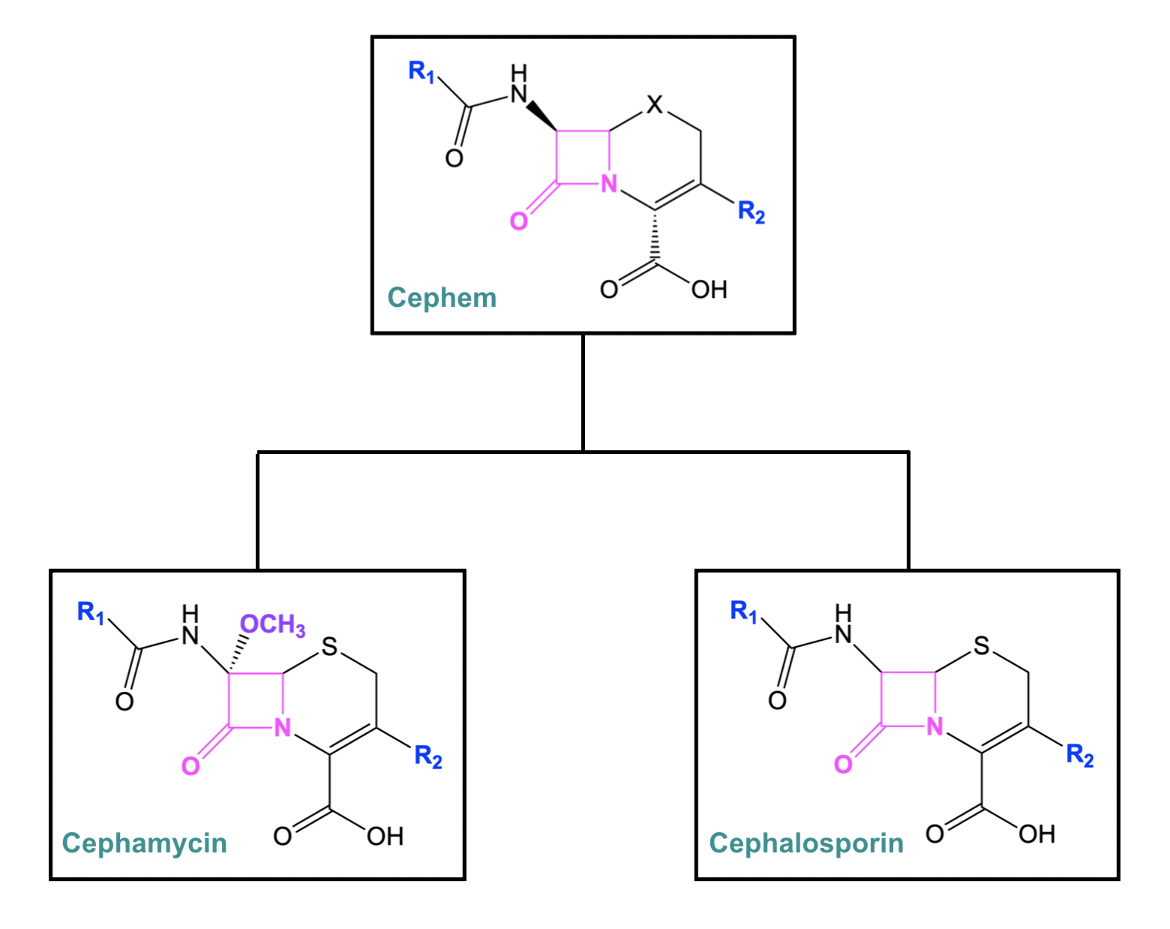
Cephems
The cephem class of antibiotics largely consists of cephalosporins and cephamycins, which contain a β-lactam ring connected to a six-membered dihydrothiazine ring. Cephems can contain either a sulfur or oxygen atom in the six-membered ring. In the case of cephalosporins and cephamycins, the ring contains sulfur. Cephalosporins are considered to be the major representative group of the cephems, as they are among some of the most potent and widely used antibacterial drugs (Fernandes et al., 2013). Except for the extra methoxy group, cephamycin is structurally very similar to cephalosporin (Figure 4).
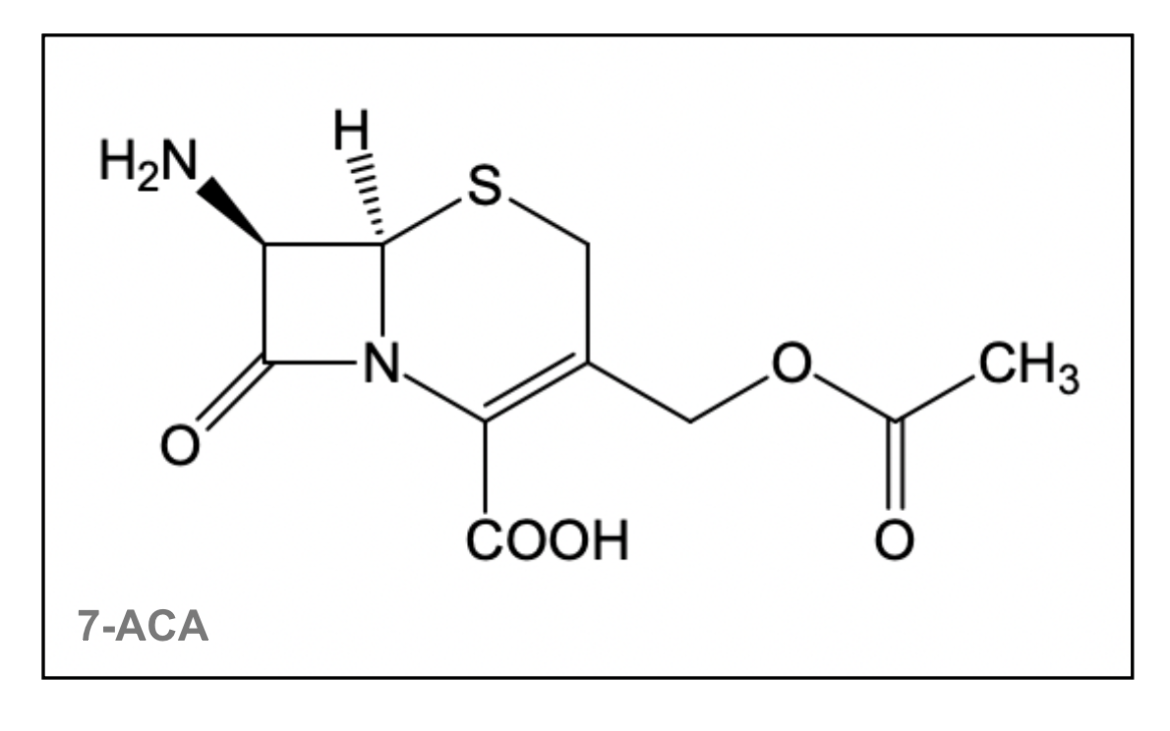
The first source of cephalosporins (Cephalosporium acremonium) was discovered in 1938, which naturally produced cephalosporin C (Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e). Using acid, cephalosporin C can be hydrolyzed to 7-aminocephalosporanic acid (7-ACA), which forms the active nucleus of cephalosporins. The addition of different side chains to this 7-ACA nucleus (shown in Figure 5) has resulted in the whole class of cephalosporin antibiotics (Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 12e).
|
| Figure 5. The 7-ACA nucleus is shared by cephalosporin antibiotics. The structure was created using ChemDraw. |
There are a large number of cephalosporin antibiotics, which makes classification of these drugs critical. These antibiotics are further divided into "generations" based on general features of antibacterial activity. Learn more about some examples of cephalosporin antibiotics including cefoxitin, ceftazidime, cefepime, and ceftaroline.
Table 1. Activity of each generation of cephalosporins against gram-positive and gram-negative bacteria. More + symbols indicate greater bactericidal activity. Table adapted from (TMedWeb).
| Cephalosporin | Gram-Positive Bacteria | Gram-Negative bacteria |
|---|---|---|
| 1st Generation e.g., Cephalexin | +++ | + |
| 2nd Generation e.g., Cefoxitin | ++ | ++ |
| 3rd Generation e.g., Ceftazidime | + | +++ |
| 4th Generation e.g., Cefepime | ++ | +++ |
| 5th Generation e.g., Ceftaroline | +++ | +++ |
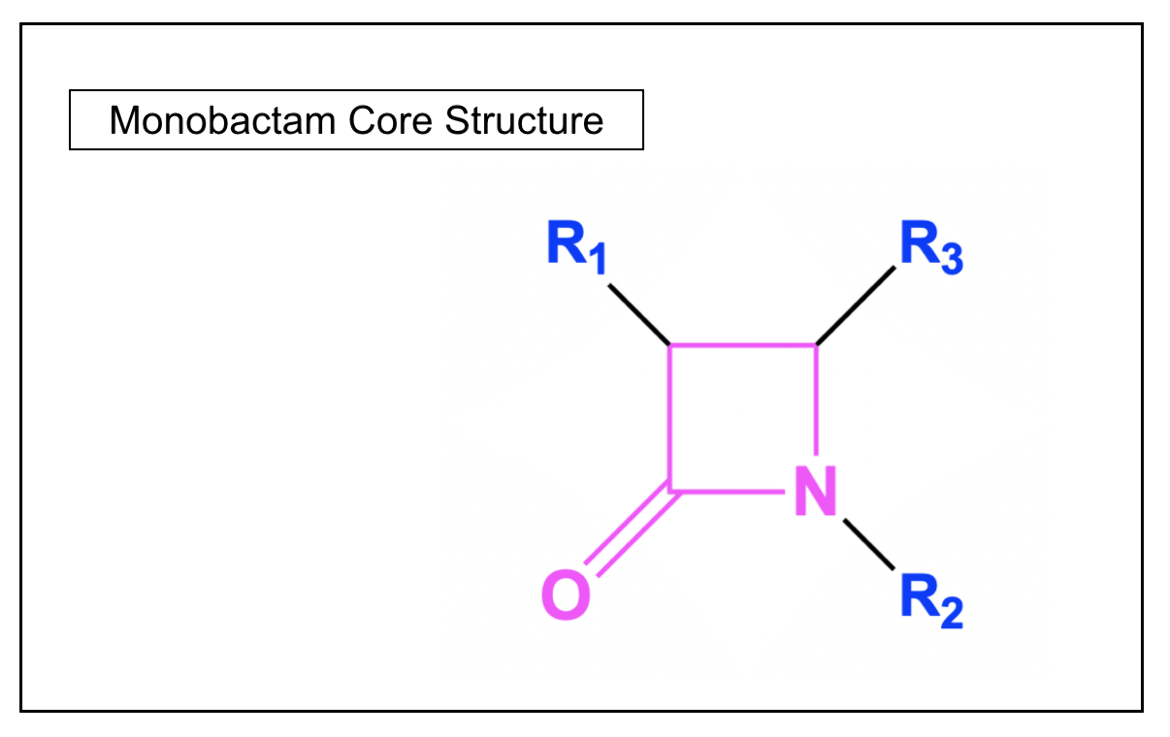
Monobactams
In contrast to the other classes of β-lactam antibiotics, monobactams are monocyclic, as the β-lactam ring is not fused to another ring structure. These synthetic antibacterial agents have been found to work only against aerobic gram-negative bacteria. The only commercially available monobactam antibiotic is aztreonam.
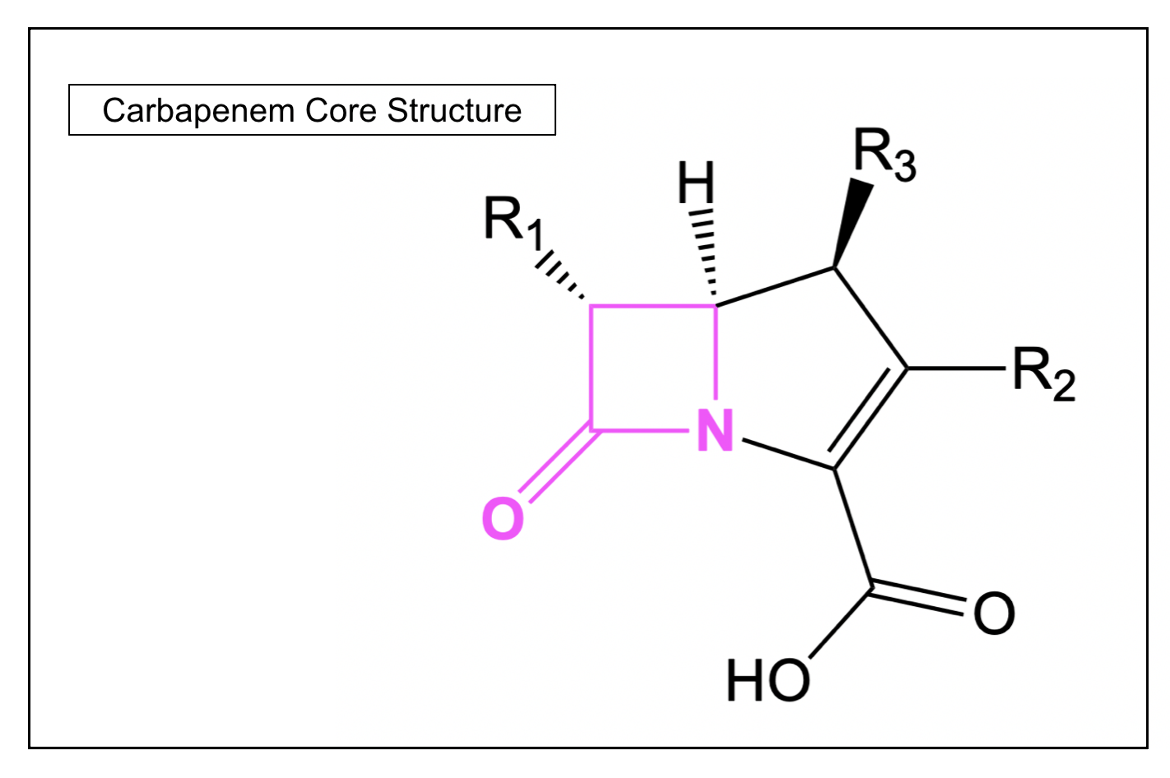
Carbapenems
Carbapenem antibiotics differ from penicillins in the fact that their β-lactam ring is fused to a five-membered ring that is unsaturated and contains a carbon atom instead of the sulfur atom. In general, this class of antibiotics has a broader spectrum activity compared to most other β-lactam antibiotics, which is why they are often used as last resort antibiotics for infections that are not responding to other narrow-spectrum antibacterial agents. One such example of a carbapenem antibiotic is meropenem.
Resistance
Many pathogenic bacteria are susceptible to β-lactams. However, some bacterial cells are not affected and can survive even in the presence of the antibiotic. This happens because these bacteria have developed different mechanisms to prevent the drug from interfering with the penicillin-binding protein function. This phenomenon is known as antibiotic resistance. As these resistant bacteria continue to grow and replicate, they cause the infection to persist and make treatment increasingly difficult.
Click here to learn more about antibiotic resistance mechanisms.
References
Brunton, L. L.; Chabner, Bruce; Knollmann, Björn C., eds. (2011) Goodman & Gilman's: The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8. 2084 pp.
Kong, K.F., Schneper, L., Mathee, K. Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS. 2010 Jan;118(1):1-36. https://doi.org/10.1111%2Fj.1600-0463.2009.02563.x
PBS, 2013, The real story behind penicillin. https://www.pbs.org/newshour/health/the-real-story-behind-the-worlds-first-antibiotic
March 2025, Gauri Patel; Reviewed by Dr. Andrew Lovering
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/antibiotics/cellwall-biosynth/pbp/blm