Rifampin Resistance
Susceptibility Testing
When possible, antibacterial substances, such as rifampin, are tested for their effectiveness against various infectious pathogens. These test results allow clinicians to choose the antibiotic likely to result in the most effective treatment of a particular bacterial infection. For instance, one such susceptibility test provides minimum inhibitory concentration (MIC) values that can then be used to identify a pathogenic bacterial strain as susceptible, intermediate, or resistant to a certain antibiotic (See Table 6).
Table 6. Minimum inhibitory concentrations (MIC) that would classify the pathogenic bacterial strain as susceptible to, intermediate, or resistant to rifampin (FDA, 2010). These values may not be the latest approved by the US FDA.
| Pathogen | MIC (µg/mL) for Susceptible (S) strains | MIC (µg/mL) for Intermediate (I) strains | MIC (µg/mL) for Resistant (R) strains |
|---|---|---|---|
| Neisseria meningitidis | ≤ 1 | 2 | ≥ 4 |
Resistance Mechanism(s)
Rifampicin resistance occurs when the antibiotic is not able to treat the infections it is intended to because the bacterial strains causing these infections have developed mechanisms to prevent the drug from functioning. These mechanisms include (CARD, 2017):
* Antibiotic target alteration through mutations
* Antibiotic inactivation
* Antibiotic efflux
Antibiotic Target Alteration – Mutations
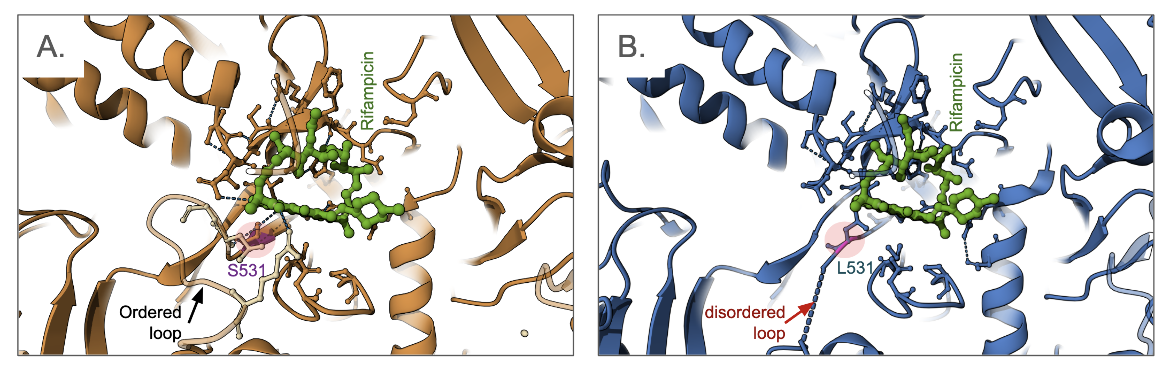
Many rifampin-resistant bacteria have mutations in the rpoB gene which encodes the β subunit of RNAP. Here we show how an example of a single amino acid change (S531L) in the β subunit of E. coli RNAP, results in one of the most clinically important rifampicin resistance mutations (Molodstov et al., 2017). The amino acid Ser 531 does not appear to play a key role in the structure and function of RNAP in the absence of rifampin. However, when comparing the structures of the native and mutant proteins in complex with the antibiotic (Figure 6), it is clear that the drug binding region in the mutant is disordered (shown as dashed lines for missing residues in the structure) and the antibiotic has weaker interactions with protein (Figure 6B). Similarly, other mutations in the RpoB protein (e.g., D516V and H526Y, not shown here) result in steric clashes and electrostatic interaction changes, all leading to weaker binding of the antibiotic (Molodstov et al., 2017).
Mutations/variations in RpoA, RpoC, and sigma factors may also lead to resistance (ARO:3000169)
Antibiotic Inactivation
Various types of rifampicin-deactivating enzymes may modify the chemical structure of the antibiotic, significantly decreasing the binding of the drug to RNAP and reducing its activity. Learn about the types of enzymatic chemical modifications of rifampin.
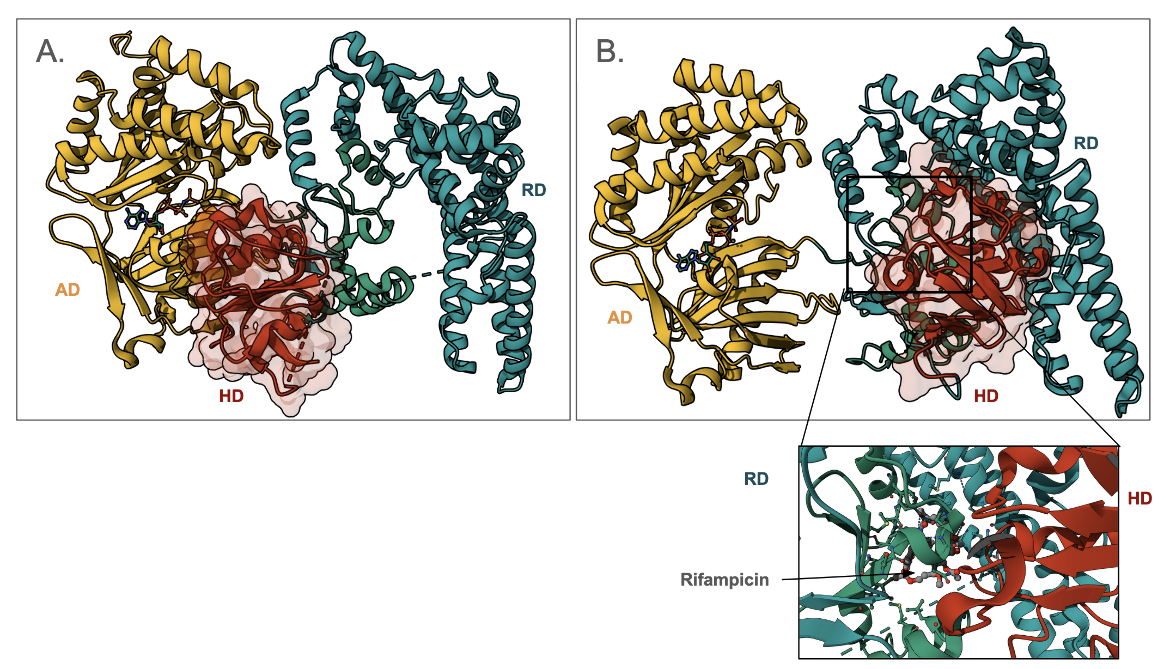
Here we show an example of a rifampin phosphotransferase from Listeria monocytogenes that phosphorylates the C21 hydroxyl of the antibiotic (Qi et al., 2016). This enzyme is shown to have 3 domains - an ATP-binding domain (AD, colored yellow in Figure 7); a rifampin-binding domain (RD, colored green in Figure 7); and the His-containing catalytic domain (HD, colored red in Figure 7). Note that the HD can swing from binding to AD and RD and is thought to carry the phosphate group from AD to RD. A close-up of the antibiotic binding site shows that the HD (colored red) helps in forming the rifampin-binding pocket.
Antibiotic Efflux
The rifamycin antibiotics, including rifampin, penetrate into the cytoplasm of bacteria. However, some resistant bacterial cells quickly remove the drug through efflux pumps. As a result, the bacteria become less susceptible to the antibacterial agent. Some examples of rifampicin resistance involving bacterial efflux pumps are described below in Table 7.
Table 7. Examples of efflux pumps that confer resistance to rifampin (ARO:3000169).
| Cause of Resistance | Description |
|---|---|
| EfpA | This major facilitator superfamily (MFS) efflux pump in Mycobacterium tuberculosis confers resistance to rifampicin, as well as some tetracyclines, lincosamides, fluoroquinolones, macrolides, and oxazolidinones. (ARO:3003955) |
| KpnEF | This small multidrug resistance (SMR) antibiotic efflux pump found in Klebsiella pneumoniae resembles EbrAB from E. coli and confers resistance to several antibiotics including rifampicin, tetracycline, streptomycin, erythromycin, and cefepime. (ARO:3004585) |
| EfrAB | This ATP-binding cassette (ABC) antibiotic efflux pump found in Enterococcus faecium confers resistance to rifampicin, ciprofloxacin, erythromycin, and quinupristin. (ARO:3003947) |
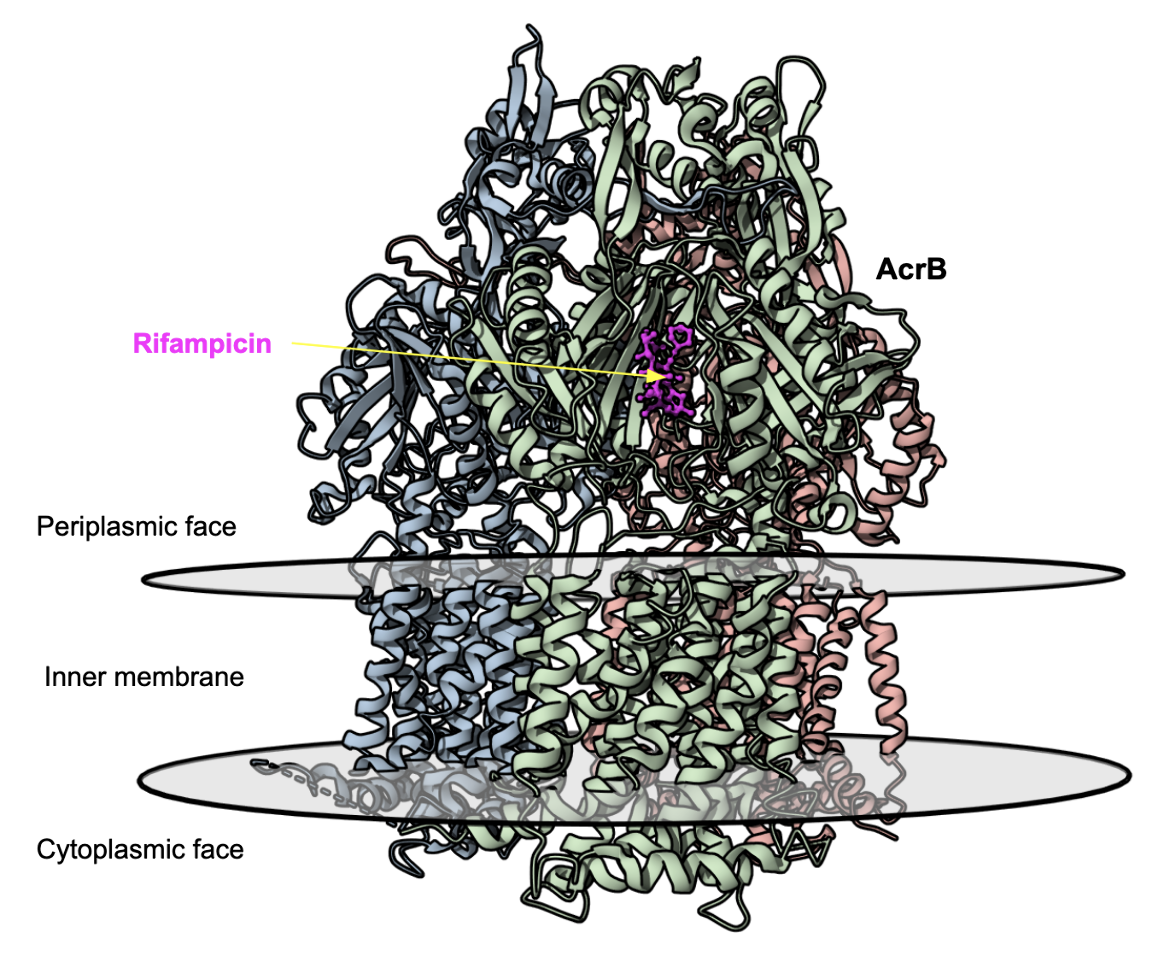
| AcrAB-TolC | This resistance-nodulation-cell division (RND) antibiotic efflux pump found in Escherichia coli forms a tripartite structure across the inner and outer membranes of gram-negative bacteria cell wall. It confers resistance to rifampicin, tetracycline, chloramphenicol, ampicillin, and nalidixic acid. (ARO:3000384). The structure of the AcrB portion of the pump is shown in Figure 8 (Nakashima et al., 2011). |
| AdeIJK | This RND antibiotic efflux pump found in Acinetobacter baumannii and contributes to resistance for many classes of antibiotics including beta-lactams, chloramphenicol, tetracycline, erythromycin, lincosamides, and rifampicin. (ARO:3000772). |
|
| Figure 8. Structures of the multidrug exporter AcrB showing the rifampicin binding pocket (PDB ID 3aob, Nakashima et al., 2011). |
Learn more about efflux proteins.
Back to the article on rifampicin.
References
Molodtsov, V., Scharf, N. T., Stefan, M. A., Garcia, G. A., Murakami, K. S. (2017) Structural basis for rifamycin resistance of bacterial RNA polymerase by the three most clinically important RpoB mutations found in Mycobacterium tuberculosis. Mol Microbiol. 103(6):1034-1045. https://doi.org/10.1111/mmi.13606
Nakashima, R., Sakurai, K., Yamasaki, S., Nishino, K., Yamaguchi, A. (2011) Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature. 480(7378):565-9. https://doi.org/10.1038/nature10641
Qi, X., Lin, W., Ma, M., Wang, C., He, Y., He, N., Gao, J., Zhou, H., Xiao, Y., Wang, Y., Zhang, P. (2016) Structural basis of rifampin inactivation by rifampin phosphotransferase. Proc Natl Acad Sci U S A. 113(14):3803-8. https://doi.org/10.1073/pnas.1523614113