Sulbactam
Drug Name
Sulbactam is a β-lactamase inhibitor antibiotic combined with other antibiotics to treat a variety of susceptible bacterial infections. Sulbactam is currently available in combination products with ampicillin.
Table 1. Basic profile of sulbactam.
| Description | β-lactamase inhibitor |
| Target(s) | β-lactamases |
| Generic | Sulbactam |
| Commercial Name | Unasyn |
| Combination Drug(s) | Unasyn (sulbactam and ampicillin) |
| Other Synonyms | N/A |
| IUPAC Name | (2S,5R)-3,3-dimethyl-4,4,7-trioxo-4λ⁶-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
| Ligand Code in PDB | 0RN, TSL (trans-enamine intermediate of sulbactam) |
| PDB Structure | 6j2b (Sulbactam bound to CTX-M-64 β-lactamase) |
| ATC code | J01CG01 |
|
|
|
Inhibitor Chemistry
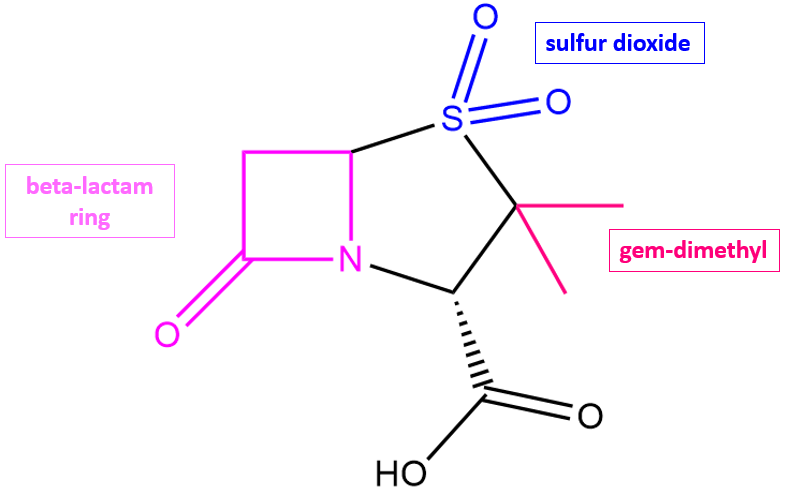
Sulbactam is a derivative of the basic penicillin nucleus and has a β-lactam ring. It possesses weak antibacterial activity, but when combined with other β-lactams like ampicillin, it increases its effectiveness.
|
| Figure 2. 2D structure of sulbactam showing the functional moieties responsible for its anti-β-lactamase activity. Structure created using ChemDraw. |
Drug Information
Table 2. Chemical and physical properties (DrugBank).
| Chemical Formula | C8H11NO5S |
| Molecular Weight | 233.242 g/mol |
| Calculated Predicted Partition Coefficient (cLogP) | -0.92 |
| Calculated Predicted Aqueous Solubility (cLogS) | -0.68 |
| Solubility (in water) | 48.5 mg/mL |
| Predicted Topological Polar Surface Area (TPSA) | 91.75 Å2 |
Drug Target
Sulbactam targets β-lactamase enzymes. These bacterial enzymes hydrolyze the amide bond of the β-lactam ring in β-lactam antibiotics, rendering them unable to inhibit their target enzyme. Learn more about β-lactamases.
Sulbactam mimics the interactions between ampicillin and β-lactamases. Therefore, when used with ampicillin, sulbactam inhibits β-lactamases from degrading the drug, acting as an effective mechanism against resistance to ampicillin by bacteria.
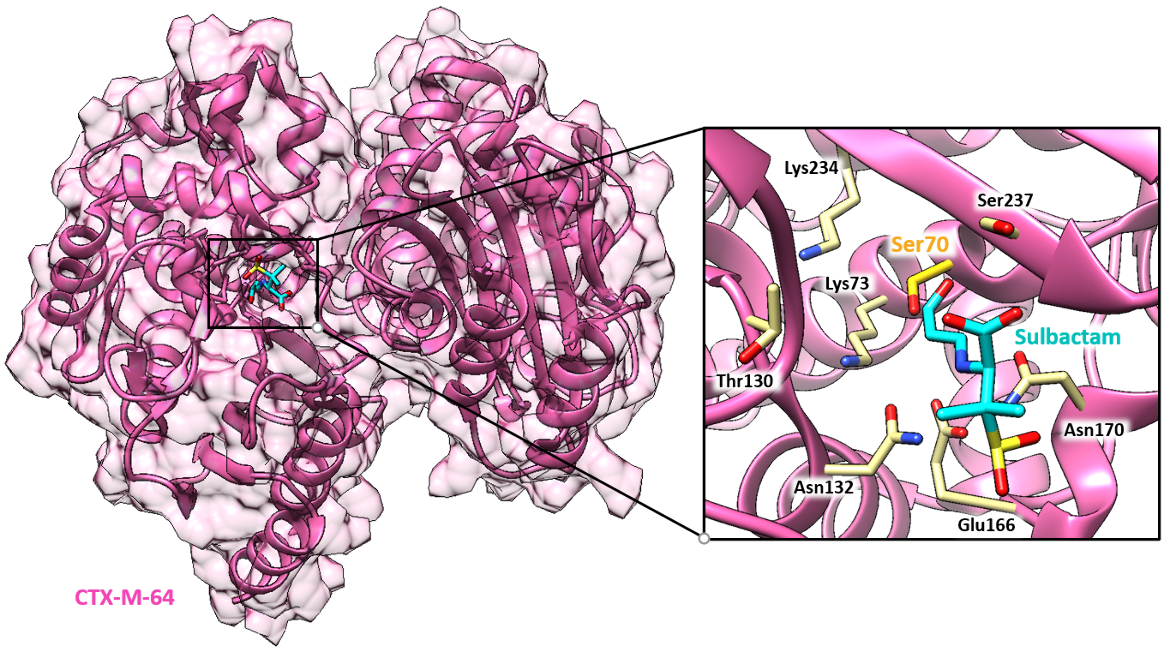
Sulbactam is an irreversible inhibitor of non-AmpC, or class C, β-lactamases. The target of sulbactam, CTX-M-64 (a class A β-lactamase), is discussed here.
Drug-Target Complex
One target of sulbactam, CTX-M-64, is an Ambler class A β-lactamase. CTX-M β-lactamases are clinically important and were named for their enhanced activity against the third generation of cephalosporin cefotaxime (Jia et al., 2017). CTX-M genes have been found on many kinds of mobile genetic elements and can be transmitted to other bacteria. Consequently, CTX-M genes have become the most prevalent extended-spectrum β-lactamase and have greater potential to spread beyond hospital environments.
CTX-M-64 cleaves the amide bond of a β-lactam drug in 2 steps: acylation and deacylation. The oxygen of the Ser70 residue in CTX-M-64 of E. coli attacks the carbonyl atom and causes acylation of the β-lactam ring to form an acyl intermediate. This initiates a cascade of proton transfers, ultimately resulting in the cleavage of the amide bond. Deacylation regenerates the catalytic serine residue, releasing the hydrolyzed antibiotic. Besides the covalent linkage with Ser70, the inhibitor forms direct hydrogen bonds (with Ser237 and Asn170), and water-mediated hydrogen bonds (with side chains of Asn104, Thr130, and backbone of Tyr105). Additionally, the Ser70 forms direct hydrogen bonds (with Lys73 ) and water-mediated ones (with Glu166).
Sulbactam blocks the active site of CTX-M-64 and prevents it from cleaving the β-lactam antibiotic with which it is combined (Figure 3).
Pharmacologic Properties and Safety
Table 3. Pharmacokinetics: ADMET of ampicillin-sulbactam.
| Features | Comment(s) | Source |
|---|---|---|
| Oral Bioavailability (%) | 1% | DrugBank |
| IC50 | N/A | N/A |
| Ki (µM) | N/A | N/A |
| Half-life (hrs) | ~ 1 hour | DrugBank |
| Duration of Action | 8-12 hours | FDA |
| Absorption Site | Gastrointestinal tract | DrugBank |
| Transporter(s) | N/A | N/A |
| Metabolism | N/A | N/A |
| Excretion | Approximately 75 to 85% of both ampicillin and sulbactam are excreted unchanged in the urine during the first 8 hours after administration. | DrugBank |
| AMES Test (Carcinogenic Effect) | N/A | N/A |
| hERG Safety Test (Cardiac Effect) | N/A | N/A |
| Liver Toxicity | Liver injury is rare and usually resolved rapidly once therapy with ampicillin-sulbactam is stopped. | LiverTox |
Drug Interactions and Side Effects
Table 4. Drug interactions and side effects of ampicillin-sulbactam.
| Features | Comment(s) | Source |
|---|---|---|
| Total Number of Drug Interactions | 50 drugs | Drugs.com |
| Major Drug Interaction(s) | bcg (Tice BCG, Tice BCG Vaccine); cholera vaccine, live; methotrexate; typhoid vaccine, live | Drugs.com |
| Alcohol/Food Interaction(s) | Food (moderate); Sodium restriction diets (moderate) | N/A |
| Disease Interaction(s) | Clostridioides difficile-associated diarrhea (CDAD) (major); Mononucleosis (moderate); Congestive heart failure, hypertension, hypernatremia (moderate); Renal dysfunction (moderate); Hemodialysis (moderate) | Drugs.com |
| On-target Side Effects | Bleeding, blistering, burning, coldness, discoloration of the skin, pain, irritation at intramuscular or intravenous injection site | Drugs.com |
| Off-target Side Effects | Diarrhea, rash, pain, tenderness, swelling of foot or leg, changes in skin color | Drugs.com |
| CYP Interactions | N/A | N/A |
Regulatory Approvals/Commercial
Sulbactam is used with β-lactam drugs to prevent the drug from being degraded by β-lactamases and increase its antibacterial properties. It is combined with ampicillin (Unasyn), amoxicillin, ceftriaxone, cefoxitin, piperacillin, and durlobactam (DrugBank, and CARD). Learn more about these antibiotics - e.g., ampicillin (Unasyn).
Links
Table 5. Links to learn more about sulbactam
| Comprehensive Antibiotic Resistance Database (CARD) | ARO: 3000587 |
| DrugBank | DB09324 |
| Drugs.com | https://www.drugs.com/mtm/ampicillin-and-sulbactam.html |
| FDA | https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050608s029lbl.pdf |
| LiverTox: National Institutes of Health (NIH) | https://www.ncbi.nlm.nih.gov/books/NBK547872/ |
| PubChem CID | 130313 |
References
Cheng, Q., Xu, C., Chai, J., Zhang, R., Wai chi Chan, E., Chen, S. (2020). Structural Insight into the mechanism of inhibitor resistance in CTX-M-199, a CTX-M-64 variant carrying the S130T substitution. ACS infectious diseases, 6(4), 577-587. https://doi.org/10.1021/acsinfecdis.9b00345
Jia, B., Raphenya, A. R., Alcock, B., Waglechner, N., Guo, P., Tsang, K. K., Lago, B. A., Dave, B. M., Pereira, S., Sharma, A. N., Doshi, S., Courtot, M., Lo, R., Williams, L. E., Frye, J. G., Elsayegh, T., Sardar, D. Westman, E. L., Pawlowski, A. C., Johnson, T. A., Brinkman, F. S., Wright, G. D., McArthur, A. G. (2017) CARD 2017: expansion and model-centric curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Research 45, D566-573. https://doi.org/10.1093/nar/gkw1004
LiverTox - Ampicillin-Sulbactam https://www.ncbi.nlm.nih.gov/books/NBK547872/
Sulbactam. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050608s029lbl.pdf
Sulbactam. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Sulbactam
Sulbactam - DrugBank. Drugbank.ca https://go.drugbank.com/drugs/DB09324
Sulbactam. Drugs.com https://www.drugs.com/mtm/ampicillin-and-sulbactam.html
April 2025, Helen Gao; Reviewed by Dr. Gregg Crichlow
https://doi.org/10.2210/rcsb_pdb/GH/AMR/drugs/OR/inh-blmase/sulbactam